The pharmaceutical industry is evolving faster than ever in 2025. Stringent regulations, rising costs, and growing demand for personalized medicine have forced pharmaceutical manufacturers to rethink their operational strategies. At the heart of this transformation lies custom pharmaceutical manufacturing ERP software—a powerful solution designed to handle the complex, compliance-heavy workflows unique to the industry.
Let’s dive into the essential benefits, features, and future trends of ERP software tailored specifically for pharmaceutical manufacturing.
What Is Custom Pharmaceutical Manufacturing ERP Software?
Enterprise Resource Planning (ERP) software centralizes data and processes into a unified system. When built specifically for pharmaceutical manufacturing, the ERP system goes beyond generic solutions—it integrates GMP compliance, batch tracking, recipe formulation, and validation processes essential to regulated environments.
Unlike off-the-shelf ERPs, custom ERP software for pharma is tailored to meet FDA, MHRA, and EMA compliance standards, offering industry-specific modules like:
- Batch & Lot Tracking
- Electronic Signatures (21 CFR Part 11)
- Quality Control & CAPA
- Regulatory Reporting
- Real-Time Inventory and Production Monitoring
Top Benefits of Custom Pharmaceutical ERP Software in 2025
1. Regulatory Compliance Made Easy
With increasing audits and scrutiny from regulatory bodies, non-compliance can cost millions. Custom ERP software simplifies compliance by:
- Automating documentation
- Supporting electronic batch records (EBR)
- Validating every process against industry protocols
2. Improved Batch Traceability and Quality Assurance
The ability to track every raw material and finished product ensures product integrity. ERP systems manage:
- Lot genealogy
- Expiry dates
- Certificate of analysis (CoA) links
With real-time alerts and automated quality checks, you prevent recalls and protect your brand.
3. Streamlined Production Planning
Pharma manufacturing often involves multi-stage processes. ERP software optimizes this by:
- Forecasting material needs
- Managing equipment usage
- Scheduling production to reduce downtime
This enables better use of resources and faster turnaround times.
4. Enhanced Inventory Control
In 2025, supply chain unpredictability remains a challenge. ERP helps by offering:
- Just-in-time (JIT) inventory management
- FIFO & FEFO inventory rotation
- Barcode scanning & RFID support
This minimizes waste, reduces storage costs, and ensures timely product availability.
5. Integrated Quality Management Systems (QMS)
Quality is the backbone of pharma. Custom ERP integrates QMS modules to manage:
- CAPA processes
- SOP documentation
- Non-conformance reporting
This leads to continuous improvement and audit readiness.
6. Cost Efficiency and ROI
Though initial implementation is an investment, long-term benefits include:
- Reduced manual labor
- Fewer errors and reworks
- Lower compliance costs
These factors contribute to measurable ROI within 1–2 years of deployment.
7. Scalability for Personalized Medicine and Growth
With the rise of personalized medicine and niche drug production, the flexibility of custom ERP systems allows:
- Modular expansion
- Support for CMOs (Contract Manufacturing Organizations)
- Adaptation to new drug formats and packaging standards
Key Features to Look for in 2025 ERP Solutions
| Feature | Why It Matters |
| GAMP 5 & FDA Compliance | Ensures validated systems for audit trails |
| Mobile Access & Cloud Integration | Supports remote work and real-time visibility |
| Multi-site Manufacturing Support | Centralizes control across facilities |
| Electronic Batch Records (EBR) | Streamlines documentation and approvals |
| AI & Predictive Analytics | Enables better forecasting and issue prevention |
| IoT Integration | Monitors equipment and environmental conditions |
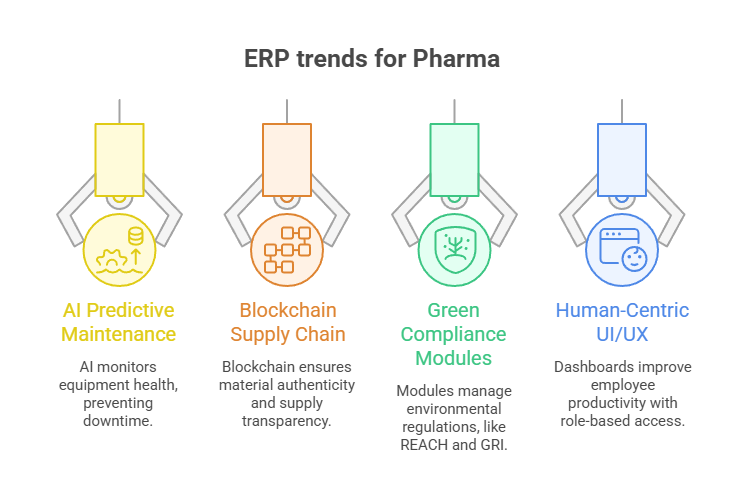
Trends Shaping ERP for Pharma in 2025
- AI-Driven Predictive Maintenance: Prevent downtime through equipment health monitoring.
- Blockchain for Supply Chain: Ensures authenticity and transparency of raw materials.
- Green Compliance Modules: Manage environmental regulations like REACH and GRI.
- Human-Centric UI/UX: Role-based dashboards for better employee productivity.
Challenges in ERP Implementation & How to Overcome Them
| Challenge | Solution |
| Resistance to Change | Provide training and involve users early in the process |
| Data Migration Complexity | Use automated ETL tools and validation protocols |
| Regulatory Validation Burden | Partner with vendors offering validation as a service |
| Budget Overruns | Prioritize features and adopt phased rollout strategies |
FAQs About Custom Pharmaceutical Manufacturing ERP Software
1. Is custom ERP better than off-the-shelf for pharmaceutical companies?
Yes, custom ERP is built for specific regulatory and operational needs, unlike generic systems that require costly customizations post-implementation.
2. How long does it take to implement a pharmaceutical ERP system?
Typically 6–12 months, depending on company size, validation requirements, and customization scope.
3. Can small pharmaceutical manufacturers afford custom ERP software?
Yes, modern cloud-based ERP options offer modular pricing and scalable features tailored for SMEs.
4. What are the most important ERP modules for pharma manufacturers?
Key modules include quality control, production planning, regulatory compliance, and inventory management.
5. Does ERP software support international regulatory requirements?
Yes, leading systems are designed for FDA, EMA, WHO, and other regional compliance standards.
6. How is data security managed in cloud ERP for pharmaceuticals?
Data encryption, user role controls, and multi-factor authentication ensure strong security protocols are in place.
Conclusion
In 2025, the need for precision, compliance, and agility in pharmaceutical manufacturing has never been greater. Custom pharmaceutical manufacturing ERP software offers a tailored solution that meets these demands head-on, streamlining operations while ensuring full regulatory compliance. Whether you're a startup launching your first facility or a global enterprise managing multi-site production, the right ERP system can be the catalyst for sustainable growth.